To complement her seminar “Could ferroelectrics improve solar energy conversion efficiencies?” Madeleine Morris has written a blog post for us on her work and around the topics she discussed. You can download the slides from her talk [PDF] as well.
Solar energy – Where are we at?
Solar is becoming an increasingly viable source of renewable energy thanks in part to both increasing device efficiencies and a rapid decrease in production costs of photovoltaic (PV) panels. Despite the drop in price, however, fabrication of silicon-based solar technologies remains a highly energy intensive and relatively expensive process. Furthermore, the efficiencies of these mature technologies, although impressive, has somewhat plateaued in the last 20 or so years.[1] The field of solar energy research, on the other hand, has not. A number of ‘emerging’ PV technologies have appeared as researchers across the world attempt to harness the Sun’s vast energy source in cost-effective, scalable and efficient ways.
Emerging PV technologies
‘Plastic electronics’ is one area which has received much attention, particularly at Imperial College London. In fact there’s a whole research centre dedicated to it: the Centre for Plastic Electronics (CPE). So-called because of the organic (that is, carbon-based) semiconducting polymers and molecules it utilises, this field concentrates on solution-processable materials. The aim is to fabricate electronic devices, such as solar cells, which are low-cost and can be produced via large-scale processing techniques, unlike their silicon-based counterparts. And it’s going reasonably well – improvements have been and are still being made. However, efficiencies of organic photovoltaics (OPVs) are on the order of 10% and so it’s just not quite efficient enough yet to allow large-scale market penetration.
Solar Fuels: the ‘other’ solar energy
Even disregarding these low efficiencies, photovoltaics alone cannot solve the world’s energy crisis – they generate electricity when the sun is up and it must either be used immediately or stored until needed. In addition, less than 20% of the world’s final energy consumption is in the form of electricity,[2] so in addition to cleaning up our electricity generation, we must consider the source of the rest of our energy consumption. Around 20% of the world’s energy usage is in transport,[3] and thus if we are to have any hope of meeting carbon emission targets we must find a clean, storable and portable alternative to fossil fuels. Electric vehicles are making excellent progress, but may not be able to cope with long-distance or flight travel. Our current infrastructure relies on burnable fuels – it could therefore be much easier and more sensible to find a way to generate a clean chemical fuel which we can integrate into our economy the way it is. Hydrogen is considered by some as the ‘ultimate’ clean fuel, and is already being utilised as a fuel in some vehicles. The problem, however, is that the overwhelming majority (~96%!) of this hydrogen is derived from fossil fuels sources, mostly steam reforming of natural gas.[4] This means that hydrogen production is currently a net contributor to carbon emissions,[5] and thus is far from being a sustainable fuel source. So, not only do we need to find a fuel that is clean upon burning, but it needs to be able to be produced in a sustainable way.
The field of solar fuels is dedicated to just that. Also known as ‘artificial photosynthesis,’ it aims to find a way to use just sunlight, water and air to make carbon-free or -neutral fuels by mimicking the solar to chemical energy conversion process which plants have been utilising for millions of years. Solar water splitting, for example, uses the Sun’s energy to break water into its elements: oxygen and hydrogen.

And there we have it – a zero-carbon fuel produced in a clean way. Alternatively, the hydrogen could be used to convert CO2 into useful carbon based molecules, which could be utilised as net carbon-neutral fuels. Unfortunately (or rather fortunately for life on Earth!), the splitting of water is a chemically challenging process: it doesn’t occur spontaneously and so we need catalysts to make it possible. Solar fuels researchers are dedicated to finding materials which are cheap, earth abundant and non-toxic which can facilitate the chemical reactions we need. We can do it, but like the organic PV field, efficiencies of this technology have much room for improvement. In fact, solar fuels conversion efficiencies are typically lower than 1%.
Fatal attraction
So why are efficiencies in both OPV and solar fuels devices so low? One of the biggest problems is a process called ‘recombination.’ In order harness the energy from solar irradiation, a photon of light must first be absorbed by a semiconductor. This creates an electron and a hole (the positive counterpart of a negative electron), the charge carriers required to convert the solar energy into electricity or chemical energy. Next comes the hard bit: we need to successfully separate the electron and hole and get them to opposite sides of a device before can we actually utilise the solar energy. The trouble, though, is that the oppositely charged carriers are coulombically attracted to each other – if they ‘feel’ each other they will recombine, and we will have lost the energy of the absorbed photon.
![]()
So in both OPVs and water splitting systems we need a driving force to pull charge carriers apart in order to make use of them. Clever ways of modifying device architecture have been developed, but tend to make fabrication processes more complex. We could also just apply an external electrical bias across the device which can be very effective for reducing recombination. But this poses logistical problems when it comes to commercial applications of these devices. And anyway, aren’t we trying to get energy out of our devices, rather than putting it in?
How could ferroelectrics help?
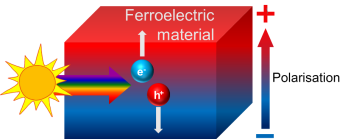 This is where a certain class of materials called ‘ferroelectrics’ come into the picture, which are the focus of my PhD research. These materials possess a permanent internal electric field within the material. A bit like a battery, one side will therefore be slightly positive and the other slightly negative. This internal electric field will drive electrons and holes in opposite directions in a material and should therefore reduce the likelihood of them recombining.
This is where a certain class of materials called ‘ferroelectrics’ come into the picture, which are the focus of my PhD research. These materials possess a permanent internal electric field within the material. A bit like a battery, one side will therefore be slightly positive and the other slightly negative. This internal electric field will drive electrons and holes in opposite directions in a material and should therefore reduce the likelihood of them recombining.
This could have big implications for PV devices as it would allow us to generate both larger currents and voltages. And for solar fuels it could be even more significant – an added difficulty of water splitting is the fact that we generate oxygen and hydrogen on the surface of a material, which will readily react with each other in a rather explosive manner. So, if we have electrons and holes going to opposite sides of the material, we’ll generate the two products in spatially distinct areas. This has the potential not only to increase efficiencies, but also make gas collection much simpler.
I’ve just told you that the internal fields in ferroelectric materials have the potential to massively reduce recombination rates in semiconductors and improve device efficiencies. But does it really work like that?
How can I help?
In my PhD research I aim to answer this question. I use a spectroscopic technique to monitor how long electrons and holes stick around for in ferroelectric materials before the recombine. By manipulating the internal fields inside the materials whilst doing this, I can investigate what effect they have on the recombination rates. The aim is to understand what goes on in these materials when we shine light on them, and use this knowledge to design more efficient devices.
So far I’ve found that recombination rates in barium titanate, an archetypal ferroelectric semiconducting material, are vastly slower than in non-ferroelectric materials with similar structures – on the order of hundreds of milliseconds rather than microseconds. What’s more, I’ve found that if I eliminate the internal fields in the barium titanate, the recombination gets several orders of magnitude faster. This strongly indicates that internal fields make a huge impact on the behaviour of photogenerated charges.[6]
What’s the catch?
Have I made a super-efficient solar cell out of barium titanate, then? Unfortunately, it’s not as simple as that. We have to consider several other factors when choosing the material(s) for our devices. Such as do they absorb visible light? Can electrons and holes move through the material easily so that they can be collected? In the case of barium titanate, and many other ferroelectric semiconductors, the answer to both of these questions is a resounding no. But it’s not all doom and gloom. Some interesting studies have shown that if you put a thin layer of a non-ferroelectric material on top of barium titanate, you can make it behave like a ferroelectric itself. So we could take a light absorbing material which usually exhibits fast recombination and put it on top of something ferroelectric and slow down the recombination. There’s also work being put into finding, or even creating, new ferroelectric materials which tick all the boxes – visible light absorbing, allow efficient transport of charges through the material and exhibit long carrier lifetimes.
Conclusion
In summary, the fields of both emerging PV and solar fuels technologies are extremely active right now. It’s an exciting time, but also a critical one. I don’t think I need to convince anyone here of the urgency of eliminating fossil fuel usage. There is a huge effort across the globe to realise this goal. To do this we must find ways to push device efficiencies up, and soon. Understanding and utilising ferroelectric materials for solar energy conversion could help to do this.
References
[1] NREL, “Best Research-Cell Efficiencies,” 2016
[2] International Energy Agency, Key World Energy Statistics 2015, 2015.
[3] EIA, “How much energy is consumed in the world by each sector?,” 2011.
[4] M. Pagliaro, A. G. Konstandopoulos, in Solar Hydrogen: Fuel of the Future, Royal Society Of Chemistry, Cambridge, 2012, pp. 1–39.
[5] P. L. Spath, M. K. Mann, Life Cycle Assessment of Hydrogen Production via Natural Gas Steam Reforming, 2001, NREL/TP.
[6] M. R. Morris, S. R. Pendlebury, J. Hong, S. Dunn, J. R. Durrant, Adv. Mater. 2016, Accepted.
Return to top

[…] fuels are reduced by a process called recombination, which is explained in a lot more detail in Madeleine’s recent blog post for Energy Futures Lab. Special materials called ‘ferroelectrics’ can help to improve […]
LikeLike